Morning Announcements
Ok, so the Staff here at FES argued that, due to the academic nature of this newsletter, they should only work according to an academic calendar. Work was progressing on the next couple of articles until early May when “summer break” was asserted.
Management scoffed, naturally.
Their resolution was rebuffed and rejected.
There were whispers of “quiet quitting” and concessions were demanded.
There was a labor strike.
Negotiations ensued.
Ultimately, the point was taken that this is a non-revenue exercise, so hopefully we’re back to a more-or-less regular schedule.
News Around the Universe
As seen elsewhere — notably, Phil Plait’s excellent
— there was a violent death in the gorgeous spiral M 101:Here’s what M 101 looks like if you get some time on the Hubble Space Telescope:

Here’s what it looks like if you’re a regular person who has a bit of time on a 16-inch telescope in Canada (like some of my gen-ed astro students!).
I happened to take this image for class purposes May 11; at that time, too miniscule to be seen here relative to the scale of its galaxy, a doomed massive star in the disk of the pinwheel was rapidly running out of fuel to keep itself inflated against the relentless crush of its own weight.

And then another image with the same telescope, taken a couple weeks later by a friend of mine who leads the collaboration:
Not hard to pick it out, even without the arrow guides. In the interim between the two images, that star desperately fused all available fuel into heavier elements all the way up to iron in a futile effort to generate enough energy to resist its gravitational implosion. Once iron was made, no more energy could be squeezed out of the core; the star rapidly collapsed and then exploded. Man, I’m telling you, I’ve been an astro-dude for 30 years, and being able to personally witness a before-and-after of gigantic events so crucial to the history of the Universe is both humbling and invigorating.
From Iron to Gold
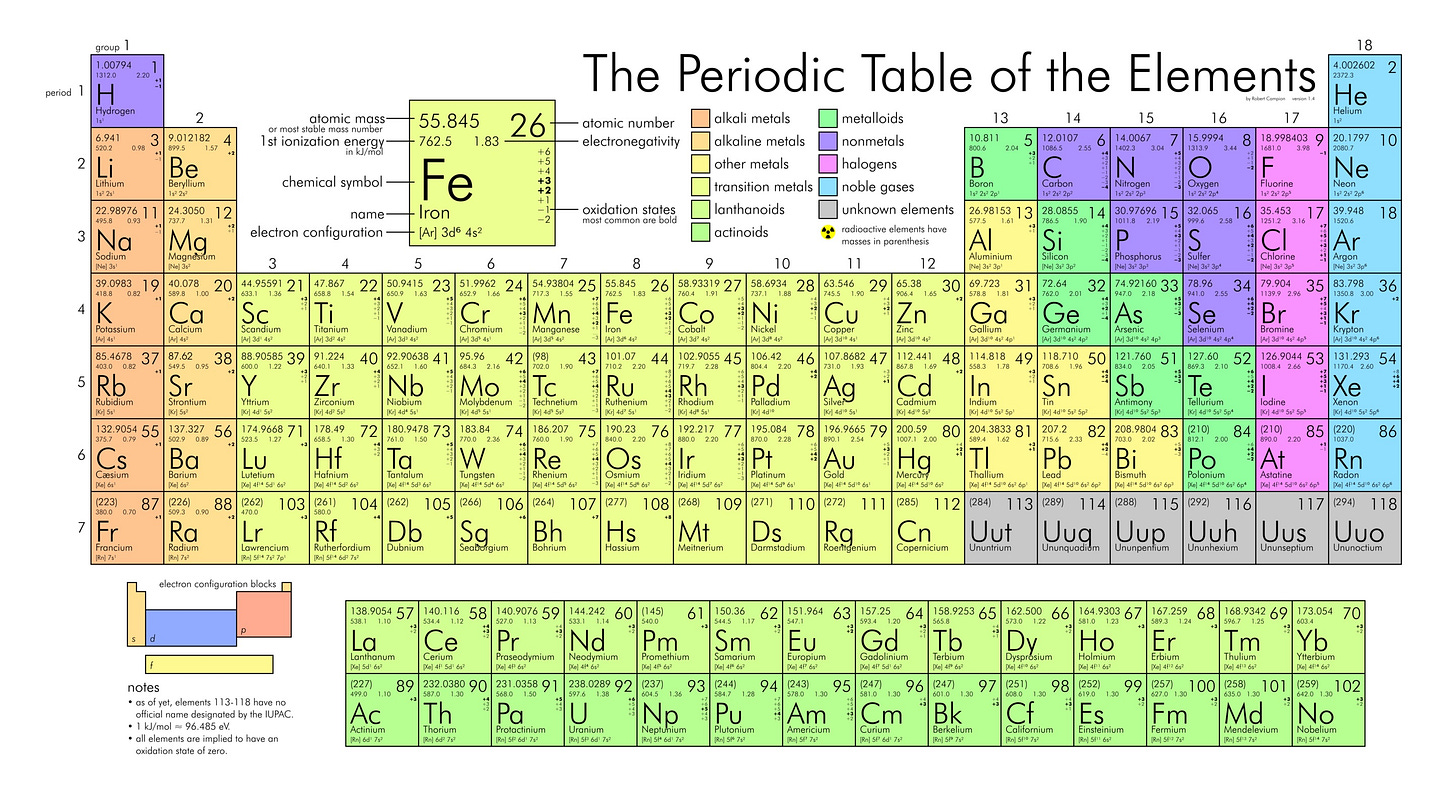
One question we always get from bright students is “if massive stars create elements up to iron (element 26) where do all the more massive ones come from?” Gold, for example, is element 79, which is well past the ability of massive stars to forge in their cores.
This is a subtle point that I think isn’t appreciated nearly enough — all the gold we know and use was already here during the Earth’s formation. It’s not being made in the core or anywhere else. So where did it come from originally? “The Big Bang”, you might imagine, maybe, but we know now that’s not right — in the very early Universe we had basically only the first 2 elements (hydrogen and helium) and a smattering of other light nuclei. [The formation of elements in the Big Bang is the subject of an upcoming post…]
All the other elements were generated as a result of stellar processes. Most of the ones up to and including iron are the result of fusion in the stars’ cores; nuclei heavier then iron form in the final titanic blasts of supernovae explosions or mergers of bizarre objects called neutron stars (which are themselves the superdense remains of massive stellar cores). That process is crazy interesting, and should probably also be the subject of a future article.
I’ll get the Staff on it.
So How Do You Want Your $Million
Anyway, as I was thinking about supernovae (and gold) I daydreamed about someone smashing that Buy Me a Coffee link at the bottom and offering me a million bucks. I mean, wire transfers are fine, but I wondered how I would actually take possession of a million dollars out on the street. Duffle bag? Backpack? And then, would it be easier to carry as a chunk of gold or as chunky fat stacks of $100 bills? I suppose the question has to do with the “dollar per gram” density of kinds of money.
Ok, well, how about gold? Right now it’s trading close to $2000 per ounce. But wait, that’s a “Troy ounce” (in the list of obscure standard units still in use, this one is right up there), equivalent to about 31 grams, so really gold is about
How much will it weigh?
or a little over 34 pounds! Not too easy to carry around, but I’d be willing to tough it out. How big would it be? Gold is one of the densest elements:
or a cube about 9.2 cm on a side. Maybe something like this:
Nice! But how would that compare with just a shoebox full of bills? Would it even fit in a shoebox? Beats me. To figure it out, I pulled out a $1 bill (I’m just a state public education employee, so I DON’T HAVE ANY HUNDREDS), and measured it to be about 15.5 cm x 6.6 cm, or about 100 square cm. I then gathered 5 of them together and weighed them on the kitchen scale. Interestingly, it weighed 5 grams. I looked it up, and indeed, US foldin’ money weighs 1 gram per bill. I had no idea.
That makes things a lot easier! Paper money has a higher “currency density” than gold, since a $100 bill would weigh 1 gram (as opposed to gold, calculated above to be about $64.30 per gram). This wasn’t obvious to me.
So if you have 1,000 bills of $100 each, that means $100,000 would weigh about 1 kg, and $1,000,000 would be 10 kg (about 22 pounds). Taking a million in bills would save my back a little, being 12 pounds lighter.
How much space would it take up? There would be 10,000 bills, each being 0.011 cm thick, so the total volume would be
or a box about 22 cm (less than 9 inches) on a side.
Tough choice — the paper money is 12 pounds lighter, but the gold takes up less room. Still, the paper money would basically fit into a shoebox, so factoring in the ease of spending of bills as opposed to trying to make change from a block of gold, I suppose I’ll accept the bills.
On Deck:
For next time, I’m working on an article related to the calculations above — it’s an astonishingly successful and simple tool in a physicist’s toolbox: Dimensional Analysis! And not just using it to convert between systems of units, but to guess at the form of complex equations. It seems to work way better than it has any right to!
If you’re a student/teacher and want to see lots of worked examples/derivations that I like to include in my classes when I teach the “standard” University Physics 1 and 2 courses, feel free to browse the (growing) collection of 150+ videos at
And if something is especially cool and you’re inclined to leave a “tip” I’m not above coffee or pizza: In fact, following Douglas Adams, I’m not above accepting coffee or pizza in the same way that the sea is not above the clouds.
Thanks for reading First Excited State! Subscribe for free to receive new posts automatically!










Thanks for the shoutout! And I once did a similar calculation, though in a different way: https://badastronomy.substack.com/p/ban-62-a-ton-of-money. 😄